当地时间5月7日,世界卫生组织宣布,由中国医药集团北京生物制品研究所研发的新冠灭活疫苗正式通过世卫组织紧急使用认证。
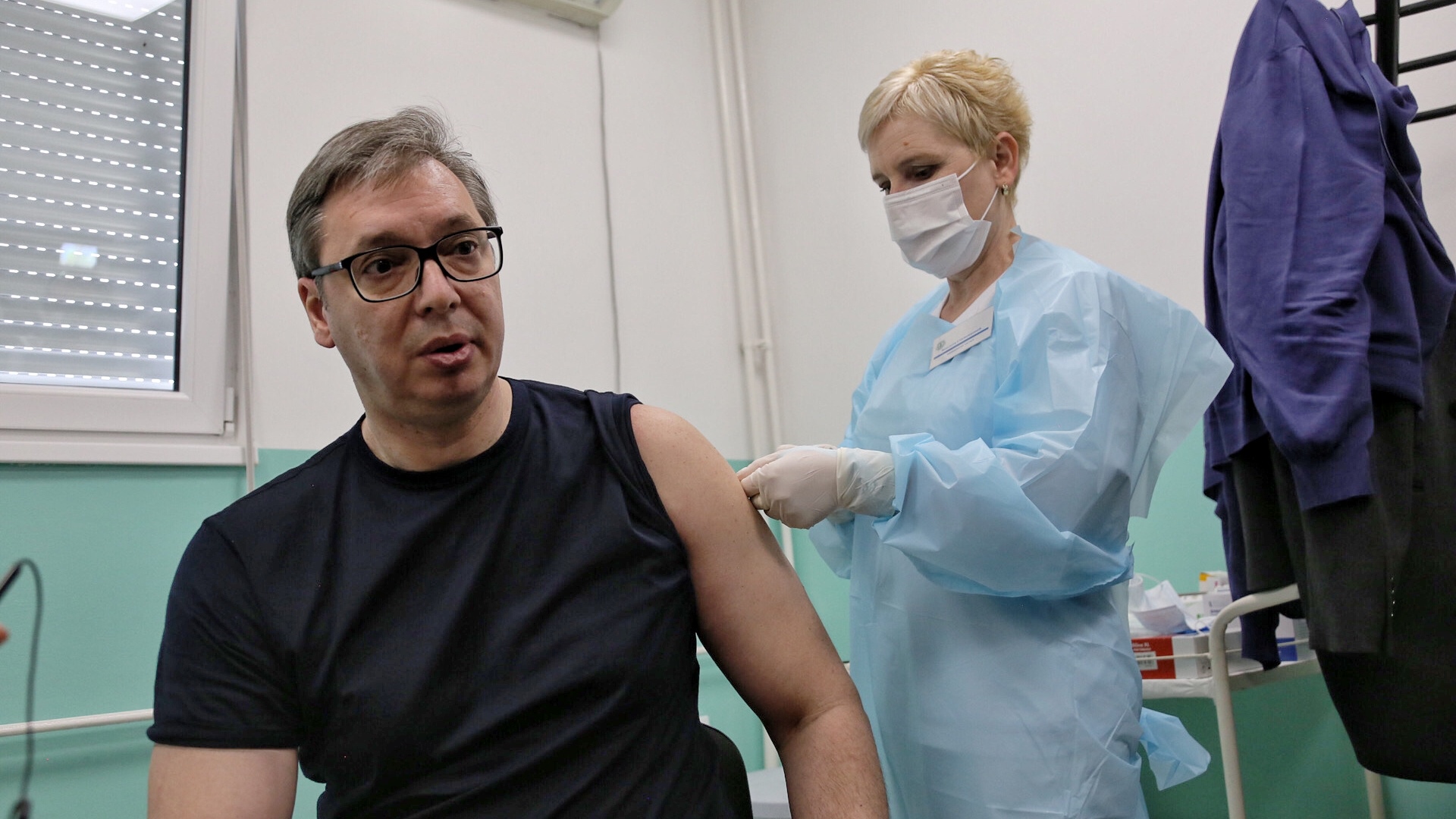
当地时间4月27日中午,塞尔维亚总统武契奇在塞南部多列瓦茨市普科瓦茨村卫生防疫站接种了第二剂中国国药集团新冠灭活疫苗。
"This afternoon, the WHO gave emergency use listing to Sinopharm Beijing's
COVID-19 vaccine, making it the sixth vaccine to receive WHO validation for
safety, efficacy and quality," said WHO Director General Dr. Tedros Adhanom
Ghebreyesus at a press briefing.
世卫组织总干事谭德塞在新闻发布会上说,世卫组织当天下午为中国国药新冠疫苗颁发了紧急使用认证,使其成为第6种获得世卫组织安全性、有效性和质量验证的新冠疫苗。
"This expands the list of vaccines that COVAX can buy and gives countries
confidence to expedite their own regulatory approval, and to import and
administer a vaccine," he said.
这扩大了世卫组织主导的“新冠肺炎疫苗实施计划”(COVAX)组合的疫苗库名单,并有助于各国加快对新冠疫苗的监管审批以及进口和接种疫苗。
【知识点】
2020年10月8日,中国同全球疫苗免疫联盟签署协议,正式加入“新冠肺炎疫苗实施计划(COVAX)”。这是中国秉持人类卫生健康共同体理念(uphold
the concept of a shared community of health for all)、履行自身承诺推动疫苗成为全球公共产品(turn
COVID-19 vaccines into a global public good)的一个重要举措。
“新冠肺炎疫苗实施计划”由世界卫生组织和全球疫苗免疫联盟、流行病防范创新联盟共同牵头成立,拟于2021年底前向全球提供20亿剂新冠肺炎疫苗(the
initial aim is to have 2 billion doses available by the end of
2021),供应给“自费经济体(higher-income self-financing countries)”和“受资助经济体(lower-income
funded
nations)”。包括我国在内的“自费经济体”需向该计划承诺为本经济体一定比例人口购买疫苗并缴纳预付款,而“受资助经济体”无须缴纳预付款,同时享受该计划补贴。
世卫组织负责获得药品和卫生产品的助理总干事玛丽安热拉·西芒当天在一份声明中表示,
The addition of the Sinopharm vaccine has "the potential to rapidly
accelerate COVID-19 vaccine access for countries seeking to protect health
workers and populations at risk".
将中国国药新冠疫苗纳入世卫组织紧急使用清单“有助于寻求保护卫生工作者和高危人群的国家加速获得新冠疫苗”。
The jab produced by the Beijing Bio-Institute of Biological Products Co.
Ltd., a subsidiary of the China National Biotec Group, is an inactivated vaccine
with easy storage requirements, which makes it highly suitable for use in
low-resource settings.
中国医药集团北京生物制品研究所研发的新冠灭活疫苗易于储存,使其非常适用于资源匮乏的环境。
It is also the first vaccine that will carry a vial monitor, a small sticker
on the vials that changes color if the vaccine is exposed to heat, letting
health workers know whether the vaccine can be safely used.
它也是第一款携带疫苗瓶监测器的疫苗,疫苗瓶上的小标签会因疫苗受热而改变颜色,便于卫生工作者判断疫苗是否安全可用。
According to the WHO's Strategic Advisory Group of Experts on Immunization
(SAGE), the Sinopharm vaccine is recommended for use in adults 18 years and
older in a two-dose schedule with a spacing of three to four weeks.
根据世卫组织免疫战略咨询专家组的意见,世卫组织建议将中国国药新冠疫苗用于18岁及以上成年人,采用两剂注射、间隔时间为3至4周。
The WHO is not recommending an upper age limit for the Sinopharm vaccine,
because reviewed data have suggested that the vaccine is likely to have a
protective effect in older persons, according to the WHO press release.
世卫组织不建议对中国国药新冠疫苗设置使用年龄上限,因为评估数据显示,该疫苗对老年人可能也有保护作用。
The WHO had previously listed the COVID-19 vaccine developed by
Pfizer/BioNTech, two versions of the AstraZeneca/Oxford vaccine, the Janssen
vaccine and the Moderna vaccine for emergency use.
此前,世卫组织已向5种新冠疫苗颁发紧急使用认证,分别是美国辉瑞制药有限公司和德国生物新技术公司联合研发的新冠疫苗、英国阿斯利康制药公司和牛津大学联合研发的两个版本阿斯利康疫苗、美国强生公司旗下杨森制药公司研发的新冠疫苗以及美国莫德纳公司研发的新冠疫苗。
重点词汇
vaccine 疫苗 ; 菌苗
According to 据 ; 按 ; 依照 ; 按照 ; 根据
spacing 间隔 ; 字距,行距 ; 以一定间隔排列 ; space的现在分词
drug and vaccine development 药品和疫苗研发
safety and efficacy research 有效性和安全性研究
a global community of health for all 人类卫生健康共同体
joint research and development of vaccines 疫苗联合研发
global public good 全球公共产品
参考来源:新华网